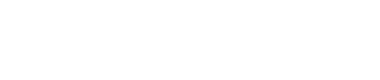Protecting your boat from corrosion is something we all have to do, but what are the implications of switching to aluminium? Vyv Cox explains how, where and when to fit your sacrificial anodes
A guide to aluminium anodes
An essential part of yacht maintenance is protecting underwater metals – such as propellers and shafts – from corrosion.
Traditionally, the most popular sacrificial anodes (which corrode instead of your valuable boat parts) have been made of zinc.

This anode for this feathering prop is small, limiting its life but a shaft anode provides extra protection
However, due to commodity prices, aluminium anodes are now 20% cheaper than their zinc alternatives.
Does this mean boat owners should switch to aluminium?
To understand the difference in properties between zinc and aluminium, and where and how to fit your sacrificial anode, it helps to understand the science behind the subject.
How anodes work
Anodes depend for their operation upon the relative positions of metals in the galvanic series. These positions are determined by measuring the voltage between the immersed metal and a reference cell composed of silver and silver chloride, also immersed in the solution, which for the purposes of this article is seawater.
A galvanic series (potential (V) vs Ag/AgCl) is thus created for all metals. In the table below, more anodic metals to the right have negative voltages compared with the cell, whereas those to the left have more positive voltages and are referred to as cathodic.
When a cathodic and anodic metal are connected together and immersed in seawater the anodic one will corrode preferentially, while the more cathodic one will be protected.
Relatively low cost sacrificial anodes are connected to our more expensive cathodic metals or, as will be seen, combinations of them.
You can see from the table that the voltages of the three commercial anode alloys (magnesium, aluminium and zinc) differ considerably from those of the general alloys which, for example, allow an aluminium saildrive leg to be protected by an aluminium anode.
The benefits of aluminium
Whilst the galvanic potentials of zinc and aluminium anodes are very similar (as shown in the table), the capacity of the aluminium version is much greater.
Thus aluminium anodes are superseding zinc ones in general use, as:
- They may be used in both salt and brackish water
- Their higher electrical capacity allows the use of smaller anodes
- The alloy remains active if exposed to air and will reactivate when re-immersed.
Zinc anodes still have some advantages but these are usually of lesser importance in yachts:
- They have better impact strength
- Their decay is more even across the anode
- The formation of zinc oxide in fresh and brackish water will inhibit their action
Magnesium alloys react rapidly in seawater and are only suitable for use in fresh water where their loss rate is reduced by lower open circuit potential.
Where to place your anode
For the protection of cathodic metals in boats there are very specific requirements for the connection and placement of anodes.
Firstly, the anode should be able to ‘see’ the surface being protected. Thus it is not possible to protect an engine with a hull anode; raw water cooled engines need an internal anode for protection.

A typical hull anode as seen on many boats. Its effectiveness in protecting the propeller is debatable when placed as far forward as this. The white deposits of zinc oxide are inhibiting its action
Secondly, the anode needs to be fairly close to the object being protected. The passage of electrons in water is not very efficient and they will fail to arrive when widely separated.
In pipelines the guideline figure is five times the diameter.
Props and shafts
Protection of propellers and shafts by anodes is possibly the largest application of cathodic protection on yachts.
The anode not only protects the shaft and propeller individually but it also protects the galvanic couple that exists between the two when made of different materials.
In its simplest form a specially shaped anode is bolted around the shaft, preferably as close to the propeller as possible.
Where the design of the drive is not suitable for a shaft anode it is usual to arrange a hull anode close to the propeller, connected electrically to it via the gearbox.

Two anodes have been fitted to this prop shaft to extend life. Ideally they could be closer to the propeller for better protection
Some shaft couplings are electrically isolating, in which the connection is made either using a copper braid bridge to bypass it or alternatively a copper brush rubbing on the shaft inside the boat.
It is good practice to test the conductivity of the arrangement with a meter between the object being protected and the anode, which should show a resistance of around 1 ohm.
Saildrives
Saildrive corrosion is a persistent source of concern for many owners.

Vyv Cox is a chartered engineer and has been sailing for more than 50 years
There are considerable differences between makers, in that Yanmar ones are electrically connected to the rest of the boat, whereas Volvo ones are totally isolated from everything else in most cases.
It is important to preserve the isolation of the Volvo ones, ensuring that no metals can bridge between the leg and the gearbox, and that the gasket is kept clean and dry.
In each case it is important to replace the anode annually, ensuring a good electrical connection on installation.
The special paint applied to saildrives is of great importance in reducing the area of the cathode and should also be checked annually.
Where anode depletion occurs more rapidly than one per season the action can be extended by hanging anodes over the side of the boat, again placing them as close to the cathode as possible. In the case of a saildrive the connection should be made to the leg itself, using insulated wire to avoid bridging the isolation.
When to replace anodes
Painting the propeller (for antifouling) has been found to double anode life by reducing the cathode area.
The basic rule is that you need sufficient anode to provide protection until the next opportunity arises to replace it with new.

The paint film on the saildrive is just beginning to break down, as shown by the small patches of corrosion. Re-painting it will avoid future problems
The actual time can vary enormously, from only a few months for some saildrive anodes to several years, particularly where the cathode surfaces, such as propellers, are painted.
In the past a rule of thumb was to replace your anode when 50% of the zinc remained. However, for aluminium this can probably be extended to only about 30% in some cases.
Chemical composition
Manufacturers add small amounts of other elements to anodes to improve their properties (see table).
However, the chemical composition is extremely closely controlled, particularly where iron is involved.
Continues below…
Stress corrosion cracking that could cost you your rig
Some types of corrosion are easy to spot, but stress corrosion cracking has the potential to weaken your rig with…
Essential seacock checks
How to check your boat for safe seacocks
Essential saildrive checks for your boat
Maintaining a saildrive is just as important as caring for the engine itself. Dennison Berwick explains how to give your…
The US Military specifies very low levels as it inhibits the action of the anode. DIY re-melting of old anodes is unlikely to comply with these specifications.
Even cleaning anodes with a steel wire brush or file may inhibit its action.
It’s therefore important to ensure that you buy anodes made to the appropriate military or marine specification.
Installing sub-standard anodes will cause increased and potentially very expensive corrosion problems.
Enjoyed reading A guide to aluminium anodes?
A subscription to Yachting Monthly magazine costs around 40% less than the cover price.
Print and digital editions are available through Magazines Direct – where you can also find the latest deals.
YM is packed with information to help you get the most from your time on the water.
-
-
- Take your seamanship to the next level with tips, advice and skills from our experts
- Impartial in-depth reviews of the latest yachts and equipment
- Cruising guides to help you reach those dream destinations
-
Follow us on Facebook, Twitter and Instagram.